The True Costs of Pandemic Explained
Summary:
The COVID-19 pandemic has posed a monumental challenge for public health experts, governments, and private organizations alike. To date, 20 coronavirus vaccines are available or in development and have been approved for use by at least one regulatory body worldwide. Each vaccine is unique in its composition and development process, offering varied levels of efficacy and safety. Sounds great. Still, the financial burden of coordination with providers and hospitals, particularly the government, has led to a massive increase in healthcare spending even during a downturn in COVID diagnoses and deaths.
COVID-19 Vaccines Explained
Vaccines developed to prevent the spread of COVID-19 have quickly become some of the most sought-after pharmaceutical products in history. This can largely be attributed to the sheer scale of the need created by the pandemic, which spurred a massive effort by drugmakers worldwide to produce safe and effective treatments on an accelerated timeline. The demand for these medicines has received significant support from governments and philanthropic organizations, who have collectively earmarked billions of dollars for vaccine procurement and distribution.
Pfizer is expected to post $101.3 billion or more in earnings from its COVID vaccines, while Johnson & Johnson could report similar earnings, having grown from 2018 ($53.7 billion) to 2019 ($82.6 billion). Most notable is the oral COVID drug Paxlovid, which likely will post $30 billion or more in 2022, and was found to successfully reduce hospitalization and/or death in patients by up to 89%! Paxlovid is used in combination with the HIV drug Ritonavir.
Why Does this Matter to You as a Payer or Provider?
Pfizer has become a significant influence on U.S. Healthcare Policy in the future. Since they became the #1 producer of COVID-19 vaccines, they have successfully raised their prices to the Biden Administration’s budget for public health. The Biden Administration, in late 2022, bought 105 million doses for $3.2 billion, which equates to an increase from $19.50 per dose to $30.47 per dose. Yes, folks, the cost of the vaccine is going up. But why?
Well, when you have an epidemic and demand is high, the price can be low or medium, depending on the emergency status of both patients and hospital staffing, but COVID-19 infection rates are on the decline.
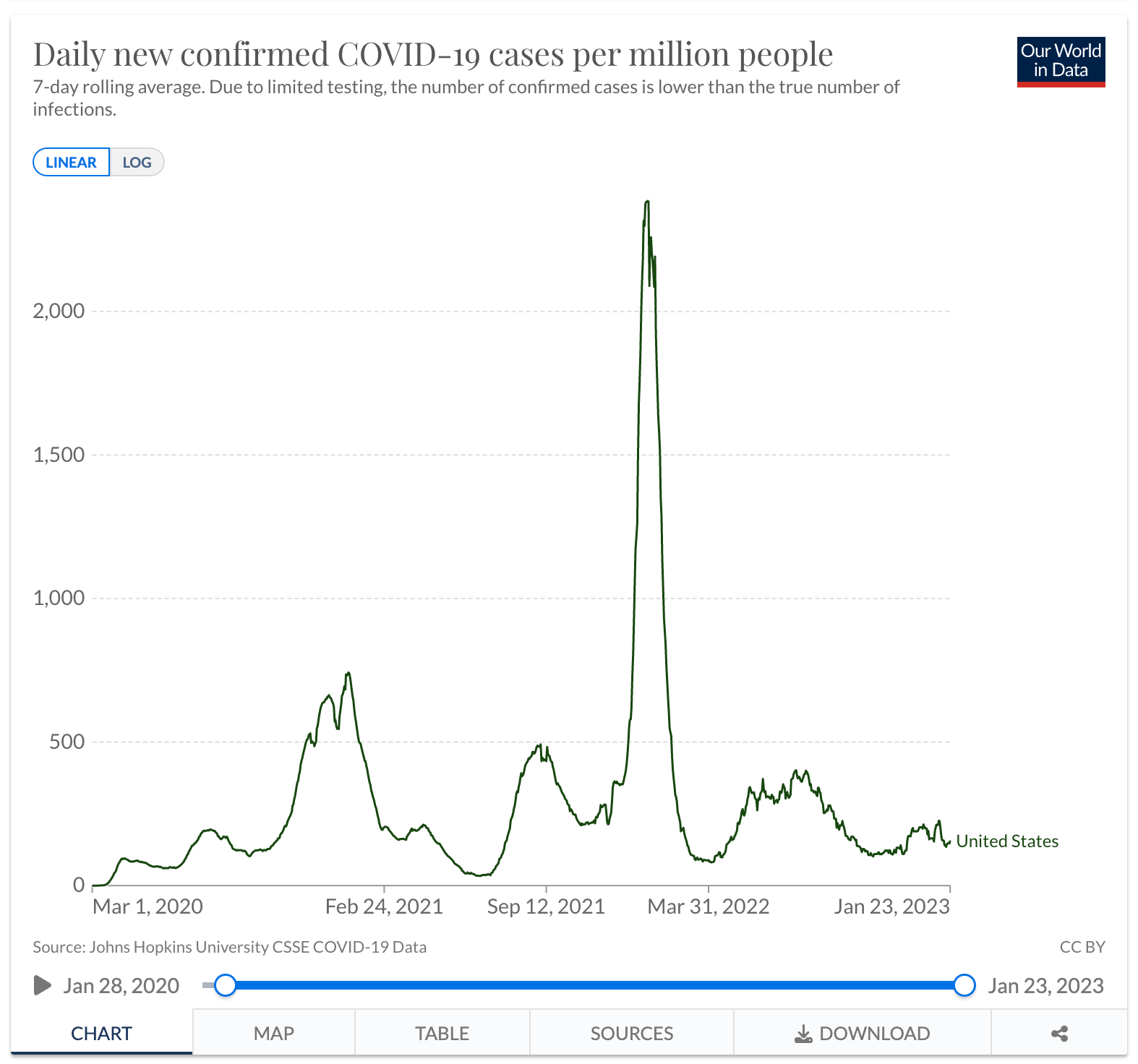
The graph below shows the new cases per day in the USA. You can see that the COVID case spikes began with a slow uptick in Q1 2020, then grew dramatically from Q4 2020 to Q2 2021, with the most significant growth in Q1 2022. Now that daily COVID cases have decreased and there are more treatment options for patients, the demand for treatment has decreased, leading Big Pharma to raise the price of the drugs in an attempt to continue its profits.
COVID-19 Statistics vs. Influenza (Flu)
From 2011 to 2019, the USA averaged 20 million to 40 million flu cases, 25,000 - 50,000 deaths per year, 10-18 million medical visits, and over 400,000 hospital visits for the flu. However, since COVID-19 entered our lives, we have had approximately 9 million flu cases in 2021-2022, rather than 20-40 million. We’ve had only 5,000 to 7,500 flu deaths. As providers and payers, we need to be able to question the legitimacy of a COVID diagnosis, which entails far more future costs at this time than a flu diagnosis.
According to both Johns Hopkins and the Mayo Clinic, over 80% of deaths result from those 65 or older, and those who have the hardest time battling COVID and its possible long-lasting effects are those with pre-existing diseases of the lung, heart, brain, cancer, kidneys, Down syndrome, and diabetes and/or obesity. So wouldn’t it make sense that COVID-19 campaigns be more focused on educating these patient populations? Wouldn’t it make more sense to spend more time with patients who fall under these conditions and see whether COVID-19 treatments and preventive care will help Americans? We don’t know, that’s why we’re asking you as providers, payers, and healthcare professionals…
Full Listing of COVID Vaccines
1. Pfizer-BioNTech COVID-19 Vaccine (BNT162b2): Developed by Pfizer and BioNTech, this vaccine is used to prevent Coronavirus disease (COVID-19). Link: https://www.fda.gov/media/144414/download
Citation: U.S Food and Drug Administration. “Pfizer’s Emergency Use Authorization for BNT162b2 mRNA COVID-19 Vaccine” December 11, 2020,
2. Moderna COVID- 19 Vaccine (mRNA -1273): Developed by Moderna, Inc., this vaccine is used to prevent Coronavirus disease (COVID-19). Link: https://www.modernatx.com/covidvaccineeauth
Citation: Moderna Press Release “Moderna Announces FDA Grant of Emergency Use Authorization for the Preventive use of its Messenger RNA (mRNA) 1273 SARS CoV 2 Vaccine against Covid 19 in Individuals 18 Years or Older” December 18th 2020
3 Johnson & Johnson Janssen COVID – 19 Vaccine Ad26 .COV2 -S): Developed by Johnson & Johnson Janssen, this vaccine is used to prevent Coronavirus disease (COVID-19). Link: https://www.jnj.com/johnson-johnson-announces-euas-for-one-dose-covid 19vaccine
Citation: Johnson & Johnson Press Release “Johnson & Johnson Announces EUAs for One-Dose COVID-19 Vaccine” March 10, 2021,
4. AstraZeneca COVID - 19 Vaccine (AZD1222): Developed by AstraZeneca and Oxford University, this vaccine is used to prevent Coronavirus disease (COVID-19). Link: https://www.astrazeneca.com/media-centre/press-releases/2021/euas-for-oxfordastrazeneca-vaccine.html
Citation: AstraZeneca Press Release “EUAs for Oxford / AstraZeneca Vaccine” February 23, 2021.
5. Novavax COVID-19 Vaccine (NVX -CoV2373): Developed by Novavax, Inc., this vaccine is used to prevent Coronavirus disease (COVID-19). Link: https://www.novavax.com/news-releases/novavax-announces-u-s-fda-grants-emergency-use-authorization-covid 19vaccine
Citation: Novavax Press Release “Novavax Announces U.S. FDA Grants Emergency Use Authorization for COVID -19 Vaccine” May 10, 2021.
6. Sinovac COVID -19 Vaccine (CoronaVac): Developed by Chinese company Sinovac Biotech Ltd., this vaccine is used to prevent Coronavirus disease (COVID-19). Link: https://www.sinovac.com/en/
Citation: Sinovac Biotech Ltd. “Sinovac CoronaVac Vaccine” May 14, 2021.
7. Sputnik V COVID-19 Vaccine (Gam -COVID -Vac): Developed by Russian developers Gamaleya Research Institute of Epidemiology and Microbiology, this vaccine is used to prevent Coronavirus disease (COVID-19). Link: https://sputnikvaccine.com/en/
Citation: Russian Direct Investment Fund “RDIF and Gamaleya Research Institute of Epidemiology and Microbiology Announce Joint Development of Vaccine Against Covid 19” August 11, 2020.
8. CanSino Biologics COVID-19 Vaccine (Adenovirus type 5 Vector): Developed by Chinese company CanSino Biologics Inc., this vaccine is used to prevent Coronavirus disease (COVID-19). Link: http://www.cansinobiologics.com/
Citation: CanSino Biologics Press Release “CanSinoBIO Announces Clinical Trial Results of its Adenovirus Type 5 Vector-Based COVID 19 Vaccine Candidate” March 24, 2020.
9. Sinopharm COVID-19 Vaccine (BBIBP-CorV): Developed by Chinese company Sinopharm, this vaccine is used to prevent Coronavirus disease (COVID-19). Link: https://www.sinopharm.com/
Citation: Sinopharm Press Release “Sinopharm Receives Emergency Use Authorization for its COVID 19 Vaccine in China” July 20, 2020.
10. Bharat Biotech COVID-19 Vaccine (Covaxin): Developed by Indian company Bharat Biotech, this vaccine is used to prevent Coronavirus disease (COVID- 19). Link: https://bharatbiotech.com/en/
Citation: Bharat Biotech Press Release “Bharat Biotech Announces Emergency Use Authorization for Covaxin, India’s First Indigenous COVID-19 Vaccine” January 7, 2021.
11. Curevac COVID-19 Vaccine (CVnCoV): Developed by German biopharmaceutical company CureVac, this vaccine is used to prevent Coronavirus disease (COVID-19). Link: https://www.curevac.com/
Citation: CureVac Press Release “CureVac Receives European Commission Authorization for its COVID 19 Vaccine” January 11, 2021.
12. Janssen-Cilag COVID-19 Vaccine (Ad26.COV2-S): Developed by Johnson & Johnson subsidiary Janssen, this vaccine is used to prevent Coronavirus disease (COVID-19). Link: https://www.janssen.com/
Citation: Johnson & Johnson Press Release “Johnson & Johnson Announces Emergency Use Authorization of Janssen COVID 19 Vaccine Ad26.COV2-S” March 8, 2021.
13. Moderna COVID-19 Vaccine (mRNA-1273): Developed by Moderna, Inc., this vaccine is used to prevent Coronavirus disease (COVID-19). Link: https://www.modernatx.com/
Citation: Moderna Press Release “Moderna Announces U.S. FDA Grants Emergency Use Authorization for mRNA-1273” December 18, 2020.
14. Pfizer-BioNTech COVID-19 Vaccine (BNT162b2): Developed by pharmaceutical giants Pfizer and BioNTech, this vaccine is used to prevent Coronavirus disease (COVID-19). Link: https://www.pfizer.com/
Citation: Pfizer Press Release “Pfizer and BioNTech Announce U.S. FDA Grants Emergency Use Authorization for BNT162b2 Vaccine Against COVID-19” December 11, 2020.
15. AZD1222 (formerly ChAdOx1 nCoV-19): Developed by AstraZeneca and Oxford University, this vaccine is used to prevent Coronavirus disease (COVID-19). Link: https://www.astrazeneca.com/
Citation: AstraZeneca Press Release “AstraZeneca Announces U.S. FDA Grants Emergency Use Authorization for AZD1222 Vaccine Against COVID-19” December 21, 2020.
16. Novavax COVID-19 Vaccine (NVX‑CoV2373): Developed by Maryland company Novavax Inc., this vaccine is used to prevent Coronavirus disease (COVID-19). Link: https://www.novavax.com/
Citation: Novavax Press Release “Novavax Announces U.S. FDA Grants Emergency Use Authorization for NVX-CoV2373 Vaccine Against COVID-19” January 22, 2021.
17. Sputnik V (Gam-COVID-Vac): Developed by the Russian Gamaleya Institute and distributed by the Russian Direct Investment Fund, this vaccine is used to prevent Coronavirus disease (COVID-19). Link: https://sputnikvaccine.com/
Citation: RDIF Press Release “Russian Direct Investment Fund Announces U.S. FDA Grants Emergency Use Authorization for Sputnik V Vaccine Against COVID-19” February 2, 2021.
18. Sinovac COVID-19 Vaccine (CoronaVac): Developed by Chinese company Sinovac Biotech Ltd., this vaccine is used to prevent Coronavirus disease (COVID-19). Link: https://www.sinovac.com/
Citation: Sinovac Press Release “Sinovac Biotech Receives Emergency Use Authorization for its COVID-19 Vaccine in China” July 17, 2020.
19. Valneva COVID-19 Vaccine (VLA2001): Developed by Austrian-French biotechnology company Valneva, this vaccine is used to prevent Coronavirus disease (COVID-19). Link: https://www.valneva.com/
Citation: Valneva Press Release “Valneva Announces Positive Regulatory Updates for its COVID 19 Vaccine Candidate VLA2001” February 22, 2021.
20. Gamaleya COVID-19 Vaccine (EpiVacCorona): Developed by the Russian Gamaleya Institute, this vaccine is used to prevent Coronavirus disease (COVID-19). Link: https://www.gamaleya.ru/en/
Citation: Gamaleya Institute Press Release “Gamaleya Institute Announces U.S. FDA Grants Emergency Use Authorization for EpiVacCorona Vaccine Against COVID-19” January 20, 2021.
COVID 19 Death Rates
There has to come a time when we as a general public need to establish COVID-19 as a treatable and not a worldly pandemic mindset. In just a single year, COVID-19 deaths have fallen from 2,179 deaths on Jan 24, 2022; to 270 deaths on Jan 24, 2023. We are better informed and have more treatment options, yet the Federal Government continues to pay more for the vaccines and boosters… PCG is not standing on one side of the vaccination or non-vaccination fence, we are just stating that with death rates declining, shouldn’t the US Healthcare System and the White House be demanding that the Vaccine price decline?
COVID Billing, Fraud, and Lessons
COVID Fraud was a blueprint for future Vaccine Fraud
COVID-19–related fraud represents the largest concentrated period of healthcare fraud enforcement in U.S. history. In April 2022, the U.S. Department of Justice announced charges against 21 defendants across nine federal districts for approximately $149 million in alleged COVID-19–related false billings. Later that year, in September 2022, federal prosecutors charged an additional 47 defendants in connection with schemes that defrauded the healthcare system, patients, and payers of more than $250 million in federal funds originally designated to support pandemic response programs, including nutrition assistance for children. Separate enforcement actions included providers charged with wire fraud, healthcare fraud, and kickback violations related to telemedicine programs that failed to comply with CMS requirements for COVID-era care delivery, exposing participants to significant criminal penalties.
This matters to providers, payers, and taxpayers because COVID-19 services were largely funded through federal emergency programs rather than patient cost-sharing. While patients incurred little or no out-of-pocket expense, the financial burden was absorbed by taxpayers and payer organizations through public funding, administrative fees, and downstream program losses. CMS and the U.S. Treasury have also warned of widespread scams involving the misuse of patient PHI and financial information to improperly access grants, stimulus payments, or healthcare benefits. These enforcement actions underscore why strong oversight, documentation controls, and fraud-prevention infrastructure remain essential long after emergency declarations expire. Suspected COVID-related scams can be reported through the FBI’s Internet Crime Complaint Center (IC3).
COVID-19 Testing and Visit Billing Without Medical Necessity
One of the most common COVID-19 fraud patterns involved billing large volumes of diagnostic tests and associated evaluation and management (E/M) services without documented medical necessity. DOJ and OIG investigations identified providers billing repeated COVID-19 PCR or rapid tests for asymptomatic patients, billing tests bundled with unrelated office visits, or submitting claims for testing that never occurred. In some cases, claims included high-level E/M codes (e.g., 99204–99215) despite minimal or templated documentation that did not support complexity, time, or decision-making.
From a payer perspective, this behavior resulted in improper payment exposure across professional, laboratory, and facility claims, often compounded by duplicate billing between labs and ordering providers. Weak linkage between diagnosis codes (e.g., Z20.822 exposure) and clinical documentation was a consistent audit finding, highlighting the importance of diagnosis-procedure alignment and frequency controls during public health emergencies.
Improper Use of COVID-19 Telehealth Billing Codes
During the Public Health Emergency, CMS expanded telehealth coverage to preserve access to care. Fraud investigations later revealed providers billing telehealth CPT codes for encounters that did not meet coverage requirements, including extremely brief calls billed as comprehensive visits, automated or scripted calls billed as physician services, or encounters performed by unlicensed staff but billed under physician NPIs. In some cases, providers billed both telehealth and in-person services for the same patient, same date, without modifier or documentation support.
These schemes frequently relied on aggressive marketing or third-party lead generation, driving volume rather than patient need. For payers, the exposure was not limited to overpayment—many cases triggered False Claims Act liability due to knowing misrepresentation of service delivery. Audits repeatedly cited missing time documentation, absent consent, and failure to meet CMS telehealth criteria despite temporary flexibilities.
Vaccination Administration and Product Billing Misrepresentation
COVID-19 vaccine fraud often involved billing for administration fees when vaccines were not actually administered, billing multiple administrations for a single dose, or misreporting place of service and provider credentials. Investigations also identified providers billing payers for vaccine product costs that were federally supplied at no charge, despite CMS guidance prohibiting reimbursement for the vaccine itself during the emergency period.
Coding errors became fraudulent when providers knowingly used incorrect CPT/HCPCS codes, billed boosters outside authorized timeframes, or submitted claims under NPIs that did not reflect who performed the service. From a payment integrity standpoint, these cases reinforced the need for tight controls around product vs. administration billing, roster billing validation, and alignment between immunization registries and claims data.
Summary
The COVID-19 pandemic created an unprecedented expansion of publicly funded healthcare services, exposing systemic weaknesses in billing controls, utilization oversight, and fraud prevention infrastructure. While emergency policies increased access to care, they also generated significant financial risk through relaxed documentation standards, rapid telehealth expansion, vaccine administration complexity, and large-scale federal funding flows with limited real-time oversight.
This article examines the true costs of COVID-era healthcare spending through the lens of billing behavior, fraud enforcement, and payer accountability. By reviewing vaccine funding structures, testing and telehealth billing practices, and documented fraud patterns, it highlights how emergency conditions translated into long-term financial exposure for taxpayers and health plans. The lessons from COVID are no longer clinical—they are operational. Strong documentation standards, consistent adjudication logic, and proactive fraud controls remain essential to prevent future large-scale program losses.
Subscribe
Only get notifications when a new article has been published
Contact Us
We will get back to you as soon as possible.
Please try again later.
Free Payer Claims Audit
Complete the form, and we'll contact you to schedule an introductory meeting and discuss our FREE 3-year claims audit to identify areas for cost containment and compliance.
Contact Us
We will get back to you as soon as possible.
Please try again later.
About PCG
For over 30 years, PCG Software Inc. has been a leader in AI-powered medical coding solutions, helping Health Plans, MSOs, IPAs, TPAs, and Health Systems save millions annually by reducing costs, fraud, waste, abuse, and improving claims and compliance department efficiencies. Our innovative software solutions include Virtual Examiner® for Payers, VEWS™ for Payers and Billing Software integrations, and iVECoder® for clinics.
Click to share with others


